|
|
|
 |
|
|
|
SAFER, CHEAPER, MORE EFFICIENT
Inside the Fourth-Generation Reactors
by Marjorie Mazel Hecht
From the Spring 2001 issue of 21st Century Science & Technology (partial text)
|
|
Artist's conception of the General Atomics 285-mw gas turbine reactor, GT-MHR.
Courtesy of General Atomics
|
|
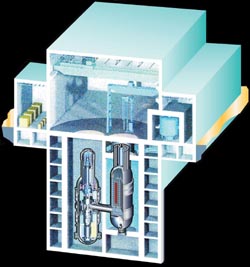 A new, fourth generation of nuclear reactors—the General Atomics GT-MHR and the South African PBMR—is ready to replace the standard reactors that have been producing power for 40 years. These new high-temperature reactors are almost 50 percent more efficient than conventional nuclear reactors, and supersafe. A new, fourth generation of nuclear reactors—the General Atomics GT-MHR and the South African PBMR—is ready to replace the standard reactors that have been producing power for 40 years. These new high-temperature reactors are almost 50 percent more efficient than conventional nuclear reactors, and supersafe.
To understand why, we’ll first review some basics of how a nuclear chain reaction is brought about and how it is controlled, so as to produce the heat that turns a turbine to generate electricity.
Fission is the splitting of the atomic nucleus of heavy elements, such as uranium, producing a quantity of heat (in the form of fast-moving particles), that is thousands of times greater than that obtained from burning an equivalent amount of coal, oil, or natural gas.1 The possibility of uranium fission, suspected by scientists for some years before, was definitively established in 1939. It was recognized right away, that such a great source of energy could serve as a peaceful energy source—or, as a powerful weapon.
When uranium fissions, each nucleus divides into two or more lighter elements (Figure 1). The fission is initiated when the uranium nucleus captures a neutral particle, called a neutron, which has been ejected by another atomic nucleus. The fission process is very fast; after a neutron is freed, it stays that way only for about 1/10,000 of a second. What makes fission so unique as a source of energy is the way it can multiply.
In fissioning, each uranium nucleus gives off two or more neutrons. This means that once the fission process is initiated, it can continue by itself, as the neutrons from each fissioned uranium nucleus trigger new fissions in nearby nuclei. This process is called a nuclear chain reaction.
|
|
|
|
|
|
|
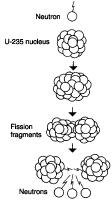 Figure 1 Figure 1
THE FISSION PROCESS
When a neutron hits a uranium-235 nucleus, the nucleus splits apart, producing two fission fragments of lighter elements, and two or three new neutrons.
The “235” in U-235 refers to the number of protons and neutrons in the nucleus. The 92 elements are listed by the number of protons in their nuclei, from hydrogen, which has 1, to uranium, which has 92. The mass number of the element is the sum of its protons and neutrons, which, in the case of fissionable uranium is 235, and in the case of nonfissionable uranium, 238. Each atom of an element has an equal number of protons (which are positively charged), and electrons (which are negatively charged), but the electrons are so tiny as to be counted as zero weight.
|
|
|
|
|
|
Chain Reactions
The first nuclear chain reaction was achieved during the wartime Manhattan Project, in December 1942. The reactor was called an atomic pile, because it was constructed with piles of graphite bricks (40,000 of them) and pellets of uranium, in what had been an underground squash court at the University of Chicago. (See Figure 2.) This was a controlled chain reaction. Nothing exploded, but heat was produced—about enough to boil a teakettle of water.
|
|
|
|
|
|
|
 Figure 2 Figure 2
THE CHICAGO ATOMIC PILE
This first U.S. “reactor,” produced a nuclear chain reaction on Dec. 2, 1942. A circular pattern of bricks of graphite (the moderator) were stacked up in layers. Alternate layers had holes drilled in them to contain uranium pellets. The control rods consisted of strips of cadmium, which were removed by hand. The shape of the pile was intended to be as close to spherical as possible, in order to capture more neutrons (by minimizing the surface-to-volume ratio).
|
|
|
|
|
|
|
Once the Manhattan Project scientists had proved that it was possible to create a chain reaction, they tackled the more difficult task of producing a nuclear bomb.
The principle of the bomb is the opposite of that of a power reactor. In the bomb, the object is to compress the nuclear fuel, surround it with explosives in a properly shaped geometry, and force the entire, hyperdense critical mass into fissioning all at once—with 280 fissions per second, in a superfast chain reaction.
In a controlled chain reaction, the configuration of uranium fuel must also achieve a critical mass. This is the amount and arrangement of uranium necessary to assure that the number of neutrons that cause fissions in other nuclei of uranium, will be greater than the number of neutrons that escape, or are captured, without fissioning. The critical mass depends on the amount of uranium, its purity, and its geometrical arrangement. (Impurities—atoms of other elements—can capture neutrons without fissioning.)
In the simplest case, it is a matter of having a large enough mass of fissionable uranium, so that the neutrons produced don’t all escape, or get captured by non-fissionable uranium and other elements, but will bump into other fissionable uranium nuclei and cause new fissions. Remember, the neutrons are neutral particles, and can whiz through the empty spaces in atoms, missing the uranium nuclei, and escaping from the mass of uranium. Critical mass involves a surface-to-volume ratio; where this ratio is minimized (as in a spherical arrangement), more fissions are likely to occur. The objective is to give neutrons less surface area from which to escape without fissioning. (In the Manhattan Project, Enrico Fermi experimented at first by bringing two lumps of uranium together, to see when it began heating up. From there, he moved to larger piles of uranium.)
|
|

Magnified photograph of a .03 inch fuel particle for the high-temperature reactor, cut away to show layers of ceramic materials and graphite surrounding a kernel of uranium oxycarbide fuel. The fuel will stay intact in its “containment building” up to 2,000°C (3,632°F).
Courtesy of General Atomics
|
|
Managing Neutrons
To sustain a chain reaction, each nucleus that undergoes fission must produce at least one neutron that will cause another nucleus to fission. The ratio of the number of neutrons in any one generation that fission, to the number of fissioning neutrons in the preceding generation is called the multiplication factor. The multiplication factor has to be more than 1.
The probability that a neutron will penetrate a nucleus to cause fission is known as the cross section of the element, and, given the sense of humor of Manhattan Project scientists, cross sections are measured in units called barns (one barn is 10-24 square centimeters—that’s a trillion-trillionth). It comes from the fact that, relatively speaking, some isotopes (like U-235) are as easy to hit as the side of a barn, compared to more difficult target isotopes.
It is possible to configure the uranium in many different ways, including very small fuel particles, arranged in such a way that the free neutrons will trigger other fissions, at a desired rate. Different reactor designs are possible, in which a controlled nuclear chain reaction is able to produce a steady and continuous amount of power, using a (comparatively) very small amount of fuel (see Note 1).
In the nuclear power reactor, depending on its type, natural uranium, or slightly enriched (3 to 5 percent) uranium is used as the fuel. The uranium ore found in nature is made up mostly of the non-fissionable isotope known as U-238. The isotope of uranium which fissions easily is U-235. But, in a sample of natural uranium ore, there is only 1 atom of the fissionable isotope present for every 140 atoms of the non-fissionable isotope. To make a bomb, the natural uranium must be “enriched” to greatly increase the ratio of fissionable U-235 to U-238.2
When uranium fissions, neutrons are produced very fast—in a ten-thousandth of a second. You might think that fast neutrons are the ones to produce fission first, but it doesn’t work that way. It was found that fast neutrons tend to be “captured,” without fissioning, by the U-238. It was only the slower neutrons that caused the U-235 to fission.
The problem then becomes, how to slow down enough of the speeding neutrons to cause enough fissions in order to sustain a controllable chain reaction. Part of the solution, arrived at during the Manhattan Project, was to combine the fuel with what is called a moderator, a lighter element of low density, so that neutrons could bounce off it and slow down, without getting captured or escaping.
For even more control of the reaction, lighter elements (like boron or cadmium) were formed into rods, which could be inserted into the reactor core to “control” the reaction rate, by absorbing neutrons. During the Manhattan Project, it was also discovered that certain proportions of fuel, and geometries of arrangement of the fuel, changed the rate of the fission reaction, by making it easier for the neutrons to cause more fissions.
What is required, however, is not only more fissions, but just the right amount to produce a steady reaction at the desired rate. Think of the problem this way: If each fission successfully produced 2 or 3 new successful fissions, the rate of fissions would multiply hundreds of times in a fraction of a second. More gradual rates are needed for a controlled reaction. . . .
|
|
|
|
|
|
|
 |
|
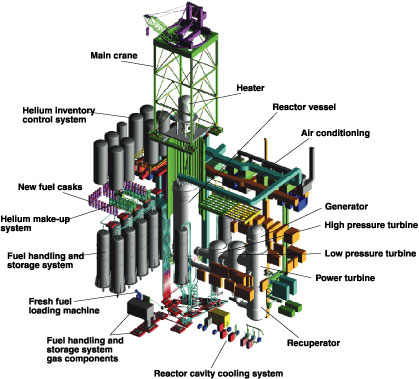
SOUTH AFRICA’S PBMR: A MELTDOWN-PROOF REACTOR
The Pebble Bed Modular Reactor (PBMR), now under development by the South African company Eskom, is shown here with its main power system and support systems.
As described in the Nuclear Report, the PBMR and its cousin, the General Atomics GT-MHR, are both helium-cooled high-temperature reactors with direct-cycle gas turbines, which eliminate the conventional steam cycle. These reactors encase the nuclear fuel in tiny ceramic spheres (instead of the familiar domed containment buildings of conventional nuclear plants).
The two designs have inherent and passive safety features, which make them meltdown proof: In brief, the reactor’s design prevents it from getting hot enough to split open the fuel particles. If there is a coolant failure, the reactor shuts down on its own, without any human intervention necessary.
The major differences in the PBMR and GT-MHR designs are in the type of fuel assembly, and the amount of power produced. The fuel for the GT-MHR is shaped into rods, while the PBMR fuel is fashioned into tennis-ball-sized “pebbles.” The PBMR is 112 megawatts electric, while the GT-MHR is 285 MWe.
PBMR fuel consists of tiny particles of uranium oxide, coated with layers of ceramics and silicon carbide, forming an impenetrable barrier, which contains the fuel. The particles are then mixed with graphite and molded into pebbles, about 310,000 of which fill the reactor vessel. An additional 120,000 graphite balls serve as moderator.
Source: Courtesy of Eskom
|
|
| Return to top |
|
|
|
|
|
|